Then calculate the number of valence electrons used in this drawing. The CO32- ion contains two CO single bonds and one CO double bond.

Resonance Structures And Calculated Mulliken Charges Of No 3 A Download Scientific Diagram
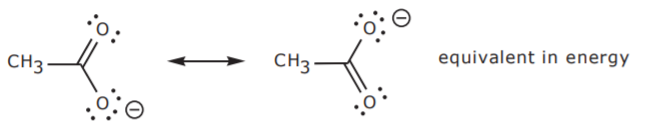
This resonance hybrid is illustrated below.

. In chemistry and physics matter is any substance that has mass and takes up space by having volume. While both resonance structures are chemically identical the negative charge is on a different oxygen in each. View solution View.
Lonone is a substance found in rose oils. I 3-Chloro-4 4-dimethyl-1-nonen-6-yne ii 4-Bromo-33-dimethyl-1-Hexen-5-yne iii R-pentane-12-diol fischer projection. Ii CH2 CH - CH CH2 iii CH2 CH - H C O.
There are many types of the carbocation is formed in different chemical reactions. The following geometries may be used. Write the CSS and draw the resonance hybrid of each of the following.
Interactive 3D display mode HC CH3 Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars including charges where needed. Draw the resonance structure of the following substance. For example we should not write a structure in which carbon has five bonds.
Ii CH2 CH - CH CH2 iii CH2 CH - H C O Solve Study Textbooks Guides. Draw the resonance structure of the following substance. By signing up youll get thousands of step-by-step solutions to your homework.
View solution Resonance structures differ only in the arrangement of _____. I CH2 CH - Cl. Drawing correct resonance structures.
Draw the chemical structure condensed of the following. Click hereto get an answer to your question Draw the resonance structures of the following compounds. The CO32- ion contains one CO single bond and two CO double bonds.
Rules for drawing resonance structures. View solution In the following the least stable resonance structure is. Draw the resonance structure of the following substance.
Draw the resonance structure of the following substance. The oxygens share the negative charge with each other stabilizing it and reducing the charge on either atom. After placing all the electrons we will have a double bond and a single bond.
Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each atom in the structure reaches an octet. In this article I will assume you are familiar with the idea of equilibrium know how to deduce the formal charge of atoms in molecules and polyatomic ions and lastly know that double bonds are shorter than single bonds. The atoms must have the same position.
Oc 0 H 120 ex cont с N O HC сн. Start your trial now. Draw the resonance structure of the following substance.
To find the resonance structure of ozone we will draw the lewis structure of ozone. In following examples arrows are used to show electrons transformation. Draw the resonance structure of the following substance.
View solution Find the substance which can form a resonance structure. The net charge on the central atom remains 1. In drawing resonance structures for a molecule we are only allowed to move electrons.
Solution for draw the chemical structure condensed of the following. Draw the Lewis structure for CO32- including any valid resonance structures. None of the above are true.
Draw the resonance structures for the following compound. To draw all resonance structures take the lewis structure we drawn by using VESPR rule. Draw the resonance structure of the following substance It is simpler than uncomplicated to create superb nail artwork for short nailsAll you need to do will be to introduce some glitter in.
Since the molecular formula is O 3 we know there are 18 valence electrons oxygen has six valence electrons as 6 x 3 18. Interactive 3D display mode. Some familiarity with the structure of organic functional groups would be helpful but is not.
Draw the resonance structures for benzene. The second-row elements C N O F can only handle up to eight electrons because of their orbitals. But to identify each resonance structures it is good to show arrows.
The resonance structure with the Formal Charge closest to zero is the most accepted structure however the correct Lewis structure is actually a combination of all the resonance structures and is not solely describe as one. Weve got the study and writing resources you need for your assignments. Draw a structure for benzene illustrating the bonded atoms.
And this means you should never place more than eight electrons on those ie. Then calculate the number of valence electrons used in this drawing. Lewis structures and resonance.
The CO32- ion contains three CO double bonds. Which of the following statements is TRUE. Draw the Lewis Structure Resonance.
First week only 499. When you are drawing resonance structures it is important to remember to shift only the electrons. Classification and Properties of Matter.
Drawing the Lewis Structure of Ozone. In resonance structures it does not require to show transformation of electrons by arrows. If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above the partial charge on each oxygen atom will be equal to - ⅔.
All everyday objects that can be touched are ultimately composed of atoms which are made up of interacting subatomic particles and in everyday as well as scientific usage matter generally includes atoms and anything made up. The positions of all nuclei must remain the same. Interactive 3D display mode.
Draw the resonance structures for benzene. Resonance structures of the nitrate ion The nitrate ion has three valid contributing structures that vary according to the placement of the electrons. 1 Do not exceed the octet on 2nd-row elements.
Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each atom in the structure reaches an octet. This is important because neither resonance structure actually exists instead there is a hybrid. Draw a structure for benzene illustrating the bonded atoms.
The single bond is active by default. Draw the resonance structures for the following compound. All of the resonance structures must be proper Lewis structures.
You must follow the octet rule. Resonance Structures are a representation of a Resonance Hybrid which is the combination of all resonance structures. Identify the oxidation.
The three possible resonance structures of NO 3 are illustrated below. I CH2 CH - Cl. Up to 256 cash back Get the detailed answer.
Resonance Structures And Calculated Mulliken Charges Of No 3 A Download Scientific Diagram
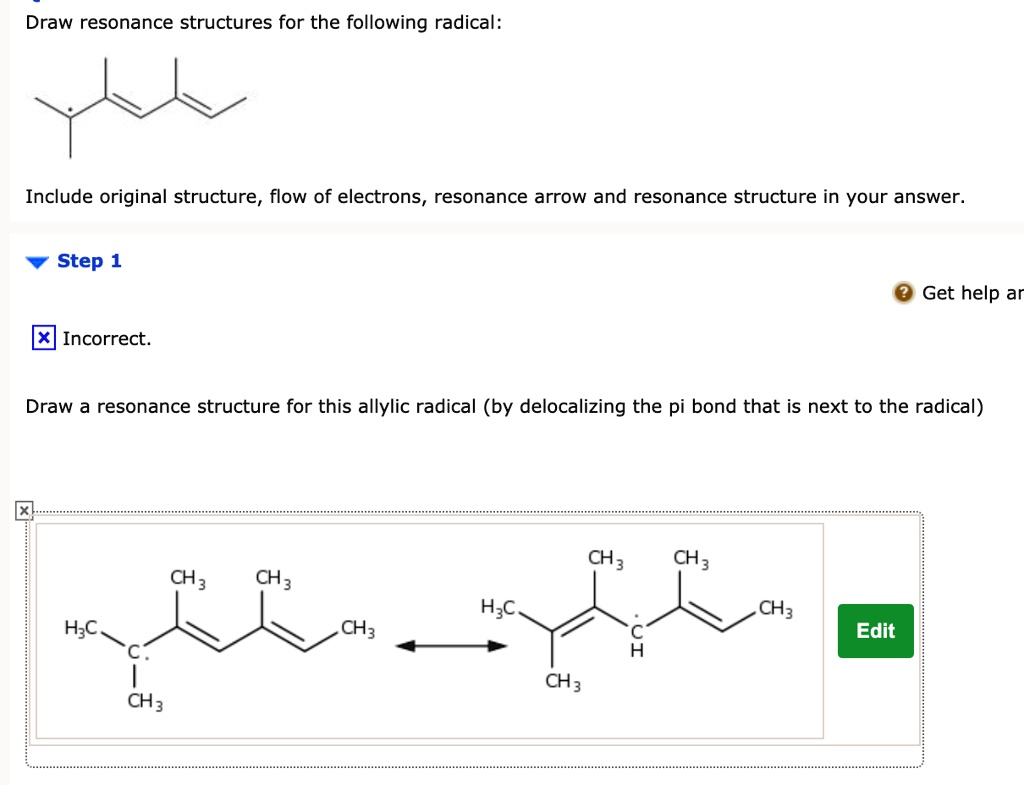
Solved Draw Resonance Structures For The Following Radical Include Original Structure Flow Of Electrons Resonance Arrow And Resonance Structure In Your Answer Step 1 Get Help Ar Incorrect Draw A Resonance Structure For

Resonance Structures Basic Introduction How To Draw The Resonance Hybrid Chemistry Youtube
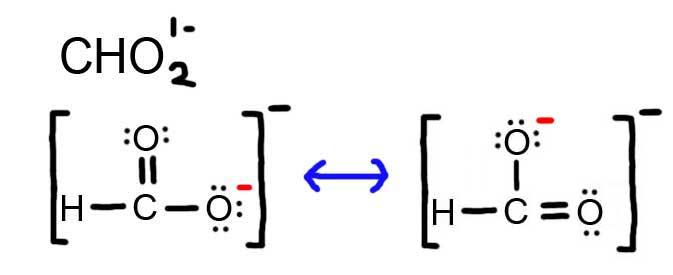
7 4 How To Draw Resonance Contributors Chemistry Libretexts

Resonance Structures And Calculated Mulliken Charges Of The Download Scientific Diagram
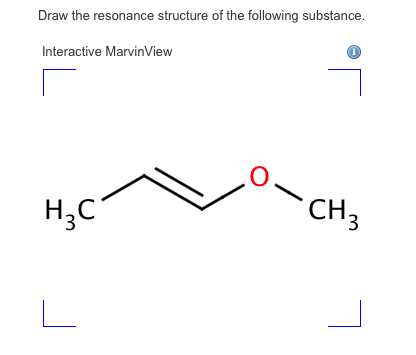
Solved Draw The Resonance Structure Of The Following Chegg Com
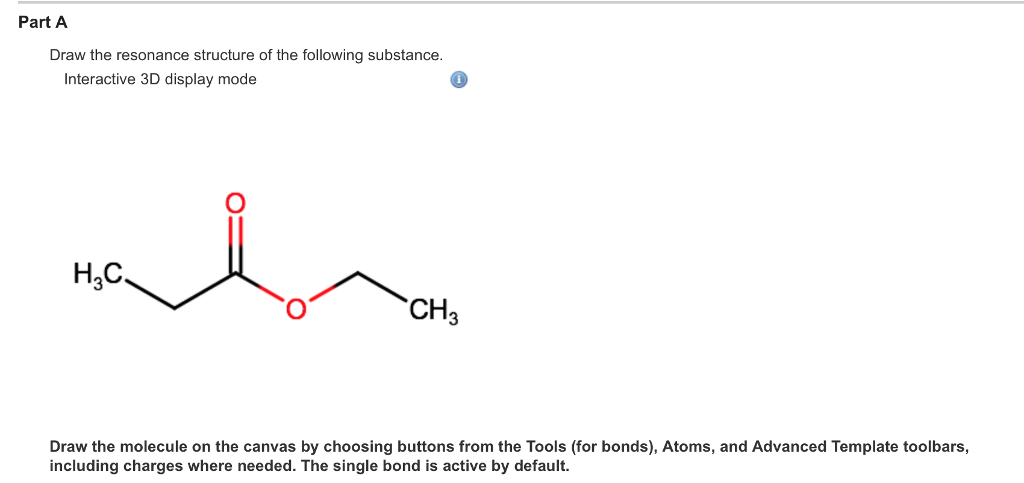
Solved Draw The Resonance Structure Of The Following Chegg Com
0 comments
Post a Comment